Biosensors have a broad array of applications from detecting analytes to characterizing and studying biomolecular interactions. Our work has had two main focal areas: (1) using optical signals, specifically, surface plasmon resonance (SPR), as transducing mechanisms, and (2) developing surface chemistries for enhanced specificity, sensitivity, and accuracy in surface-enhanced Raman scattering (SERS) and SPR biosensors. Recently, we have begun working to develop biocompatible conducting polymers for organic electrochemical transistor (OECT) biosensors.
Understanding and design plasmonic nanostructures for tunable, strong SERS substrates.
Because of its molecular specificity and high sensitivity, SERS has emerged as a powerful analytical and sensing technique with broad applications. Using plasmonic nanostructures as SERS-active substrates is one of the frontiers in this field, because localized SPR induces extremely strong local electric fields, which in turn provide electromagnetic enhancements that dramatically impact the SERS effect. Our goal has been to fundamentally understand the physical origin of this localized SPR, enabling us to rationally design plasmonic nanostructures for specific applications.
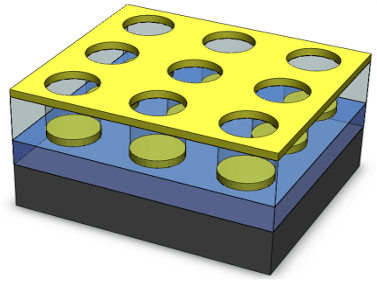
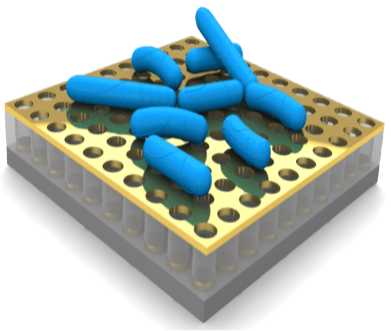
- We have developed unique quasi-three-dimensional plasmonic nanostructure arrays (Q3D-PNAs) that have a strong, tunable local electric field at the dielectric/metal interface of either the top gold nanohole or bottom gold nanodisk, providing a means for sensitively detecting large analytes (e.g., bacteria) or small targets (e.g., molecules, proteins and viruses), respectively.
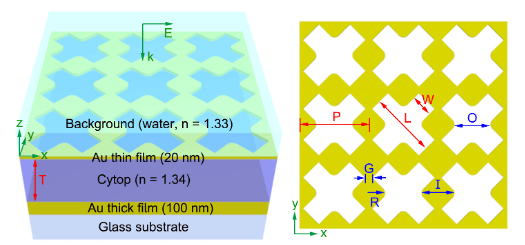
- We have proposed a concept for long-range SERS and designed the plasmonic nanostructures that could extend the physical reach of the strong local electric field by more than twenty times above the metal surface, compared to the conventional SERS substrates.
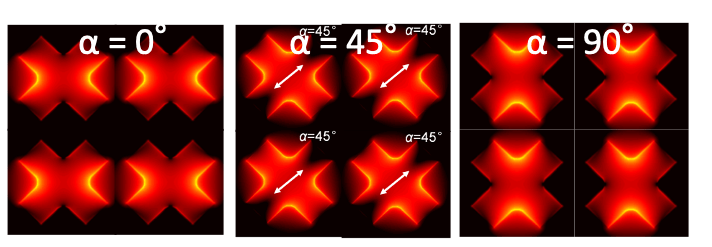
- We have developed x-shaped Q3D-PNAs that exhibit the polarization dependent of the strongest local electric fields, offering a unique tool for studying inhomogeneous samples, attached to their tops, via polarization dependent, enhanced Raman scattering.
Development of surface chemistries to enable specific, sensitive detection of small molecules in complex media.
Detection using SERS is typically carried out on bare metal surfaces because it is a near-field effect. However, non-specific adsorption frequently occurs that significantly reduces the detection performance. In addition, the intrinsically small Raman scattering cross-sections and low surface affinity of biomolecules make it very difficult to achieve clinically relevant limits of detection. Therefore, our goal is to develop surface chemistries that enable sensitive, specific, and accurate detection of analytes from complex media.
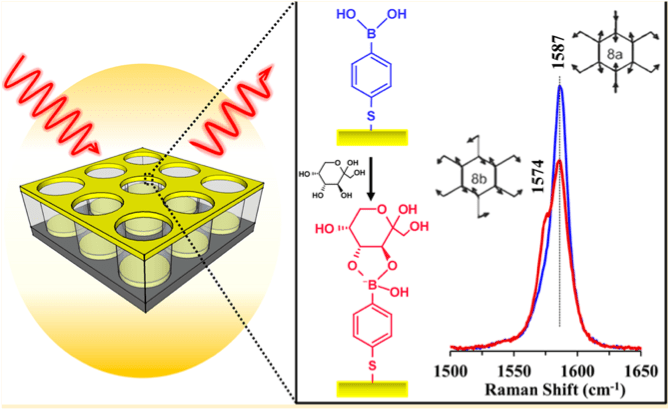
- We proposed a new conceptual strategy that indirectly detects analytes by monitoring the SERS signal change of probe molecules and identified a family of thiophenol-based probe molecules and immobilized them onto the SERS substrates to realize specific, sensitive, and reproducible detections.
- We developed a stealth surface modification for SERS substrates by forming a mixed self-assembled monolayer, consisting of probe and non-fouling molecules with strong and weak Raman signals, respectively, to enable the sensitive and specific detection in protein solutions.
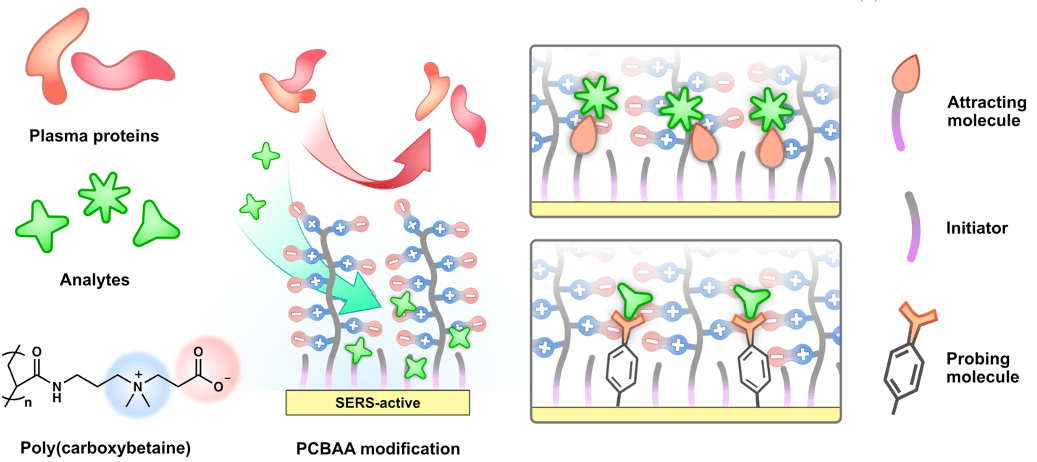
- We further developed a hierarchical zwitterionic modification for SERS substrates using a two-layer architecture: a mixed self-assembled monolayer of probe/ligand and polymerization initiator composing the first layer on the metal surface, and a zwitterionic non-fouling polymer making up the second layer, to enable real-time drug monitoring from blood plasma.
Rapid distinguish and identification of pathogens with high reproducibility and sensitivity.
The specifically designed Q3D-PNAs, exhibiting strong and uniformly distributed local electric fields, allows us to conduct rapid detection of pathogenic bacteria using SERS. We aims to combine machine learning and SERS imaging to achieve fast, high throughput characterization and detection of pathogens with broader applications from environmental monitoring, food safety to medical diagnosis.
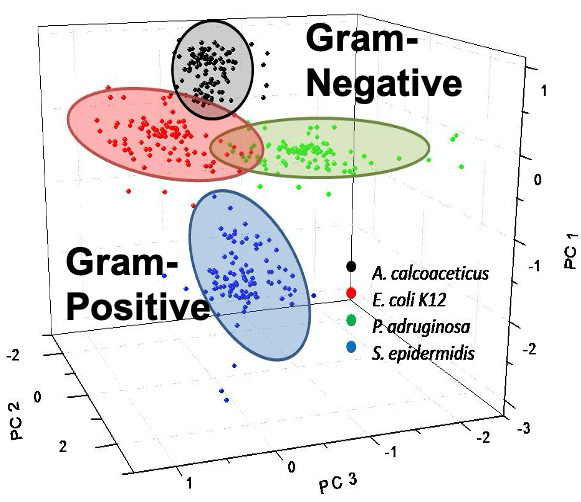
- We have demonstrated that gram-negative and gram-positive bacteria can be rapidly distinguished by applying principal component analysis to the SERS spectra because of the difference in their cell membranes.
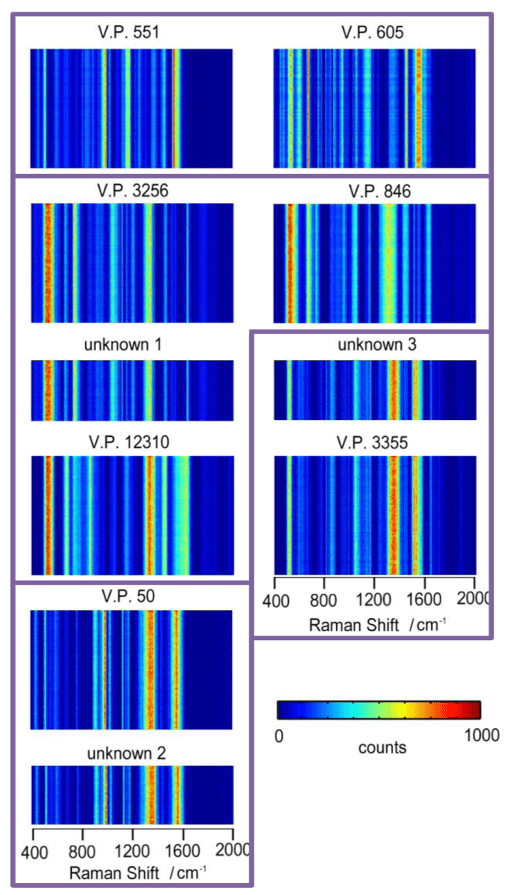
- We have generated SERS bar-coding for rapid identification of marine bacteria Vibrio Parahaemolyticus with the strains that are originated from clinical and environmental sources in Washington, U.S.A. and represent four genetically similar clades.
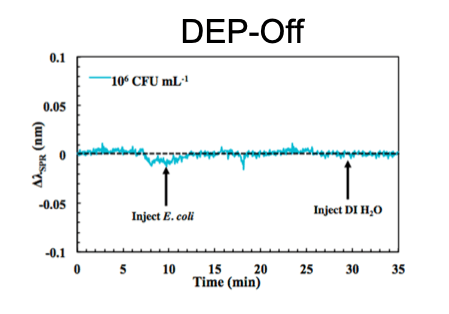
Integration of dielectrophoresis into SPR biosensors.
The primary advantages of SPR biosensors include label-free, real-time, molecular-level detection and characterization of ligand-receptor interactions. The SPR wavelength shifts when analytes bind to the gold sensing surface at the bottom of a flow channel, but detecting bacteria using this method is often compromised by the diffusion-limited mass transport of bacteria to the sensing surface. To address this, we developed dually functional interdigitated electrodes that could sustain SPR and increase bacterial mass transport via the external application of dielectrophoresis. We successfully demonstrated a limit of detection improvement of five orders of magnitude and specific detection of E. coli by applying the techniques of dielectrophoresis, surface functionalization, and secondary antibody amplification. This work indicates the great potential of incorporating dielectrophoresis into SPR biosensors for rapid, sensitive, and specific bacterial detection, with broad applications in areas including biomedical diagnostics, environmental monitoring, food safety, and homeland security.
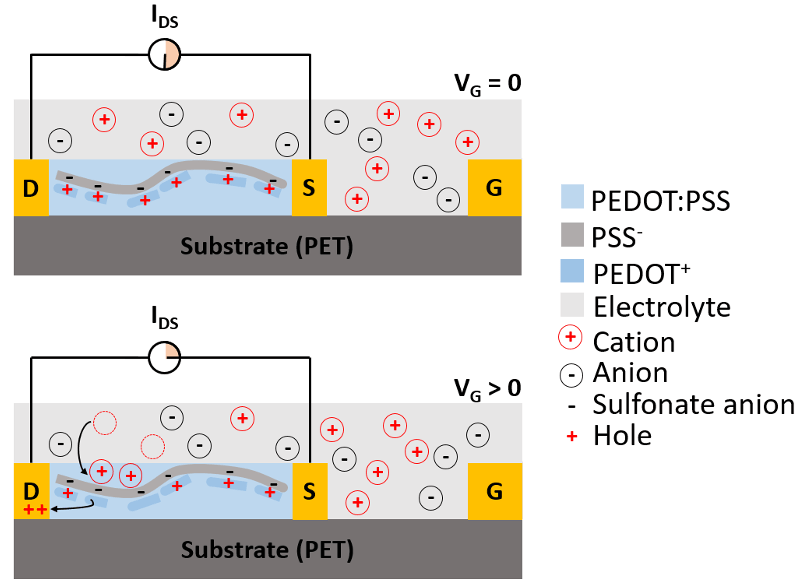
Development of functional biocompatible conducting polymers for organic electrochemical transistor (OECT) biosensors.
Recently, we have initiated efforts into developing a new type of biosensor based on OECTs, which offer several advantages such as simple electrical readouts, low operating voltages, inherent signal amplification, straightforward miniaturization, and facile fabrication on flexible substrates such as paper. These make OECTs excellent candidates for wearable biosensors as well as for remote and disposable biosensors. Poly(3,4-ethylenedioxythiophene), doped with poly(styrenesulfonate) (PEDOT:PSS), is commonly used as a conducting polymer between the OECT source and drain because of its high conductivity, easy processing, and commercial availability. However, PEDOT:PSS presents some limitations, mostly originating from a lack of PEDOT functionality and low biocompatibility of PSS. We aims to develop biocompatible conducting polymers to advance the applications of OECTs for biosensors.